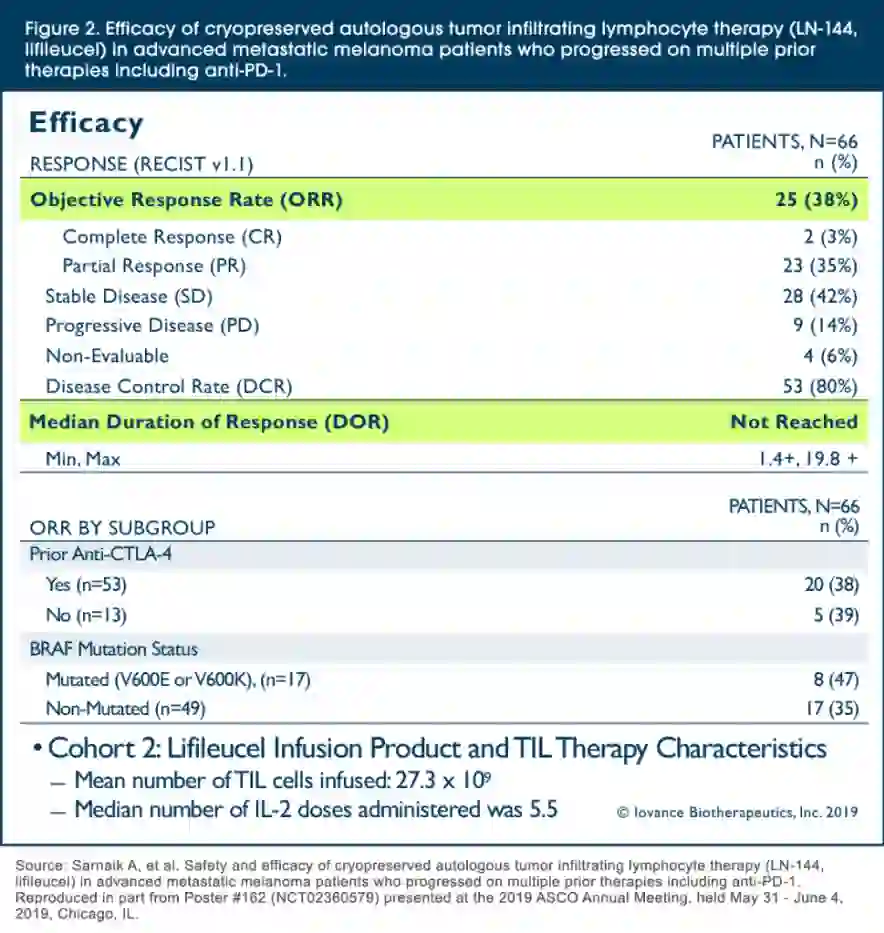
Esophageal adenocarcinoma with any component of signet ring cells portends poor prognosis and response to neoadjuvant therapy - ScienceDirect
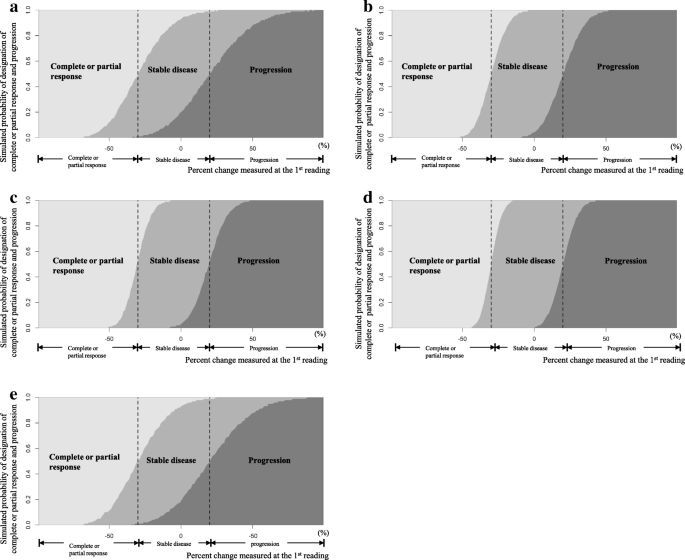
Simplifying the Derivation of Best Overall Response per RECIST 1.1 and iRECIST in Solid Tumor Clinical Studies

Response rates Overall response rate and depth of response according to... | Download Scientific Diagram
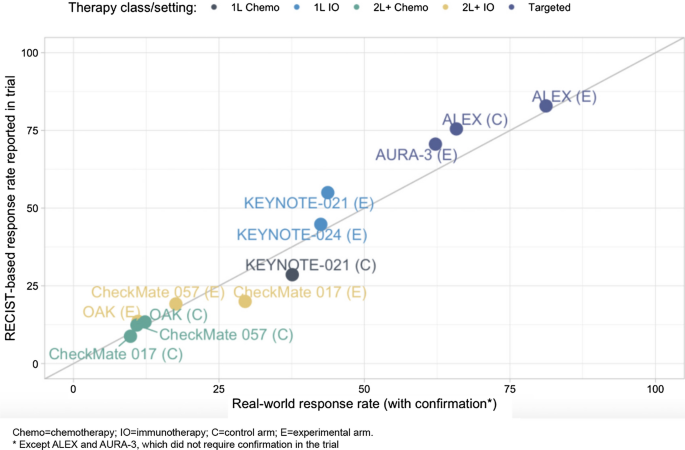
Characterization of a Real-World Response Variable and Comparison with RECIST-Based Response Rates from Clinical Trials in Advanced NSCLC | SpringerLink

Overall vs. Progression-Free Survival, Response Rates: Oncology Terminology 101 (by Oster Oncology) - YouTube
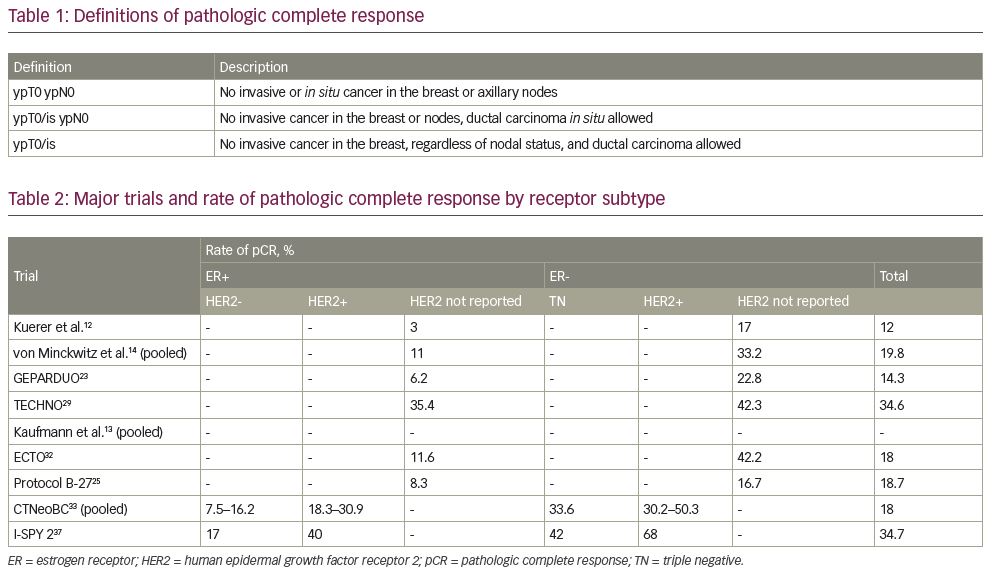
Physical exercise during neoadjuvant chemotherapy for breast cancer as a mean to increase pathological complete response rates: Trial protocol of the randomized Neo-ACT trial | PLOS ONE

Characterization of a Real-World Response Variable and Comparison with RECIST-Based Response Rates from Clinical Trials in Advanced NSCLC | SpringerLink
KAZIA PRESENTS FURTHER PAXALISIB AND CANTRIXIL DATA AT AACR, REINFORCING POSITIVE EFFICACY SIGNALS FOR BOTH DRUGS

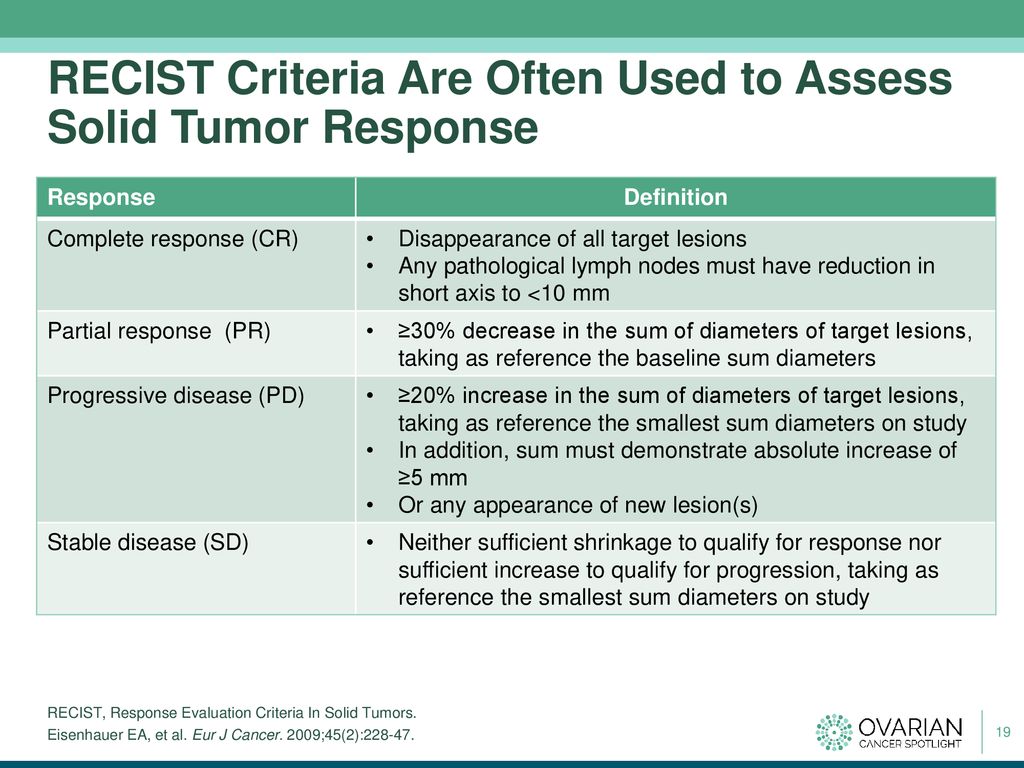
Overall Response Rate, Progression-Free Survival, and Overall Survival With Targeted and Standard Therapies in Advanced Non–Small-Cell Lung Cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses | Journal of Clinical Oncology
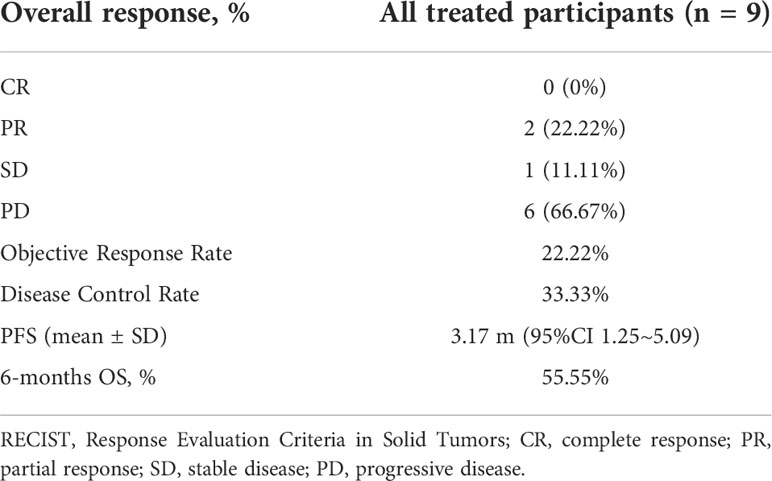
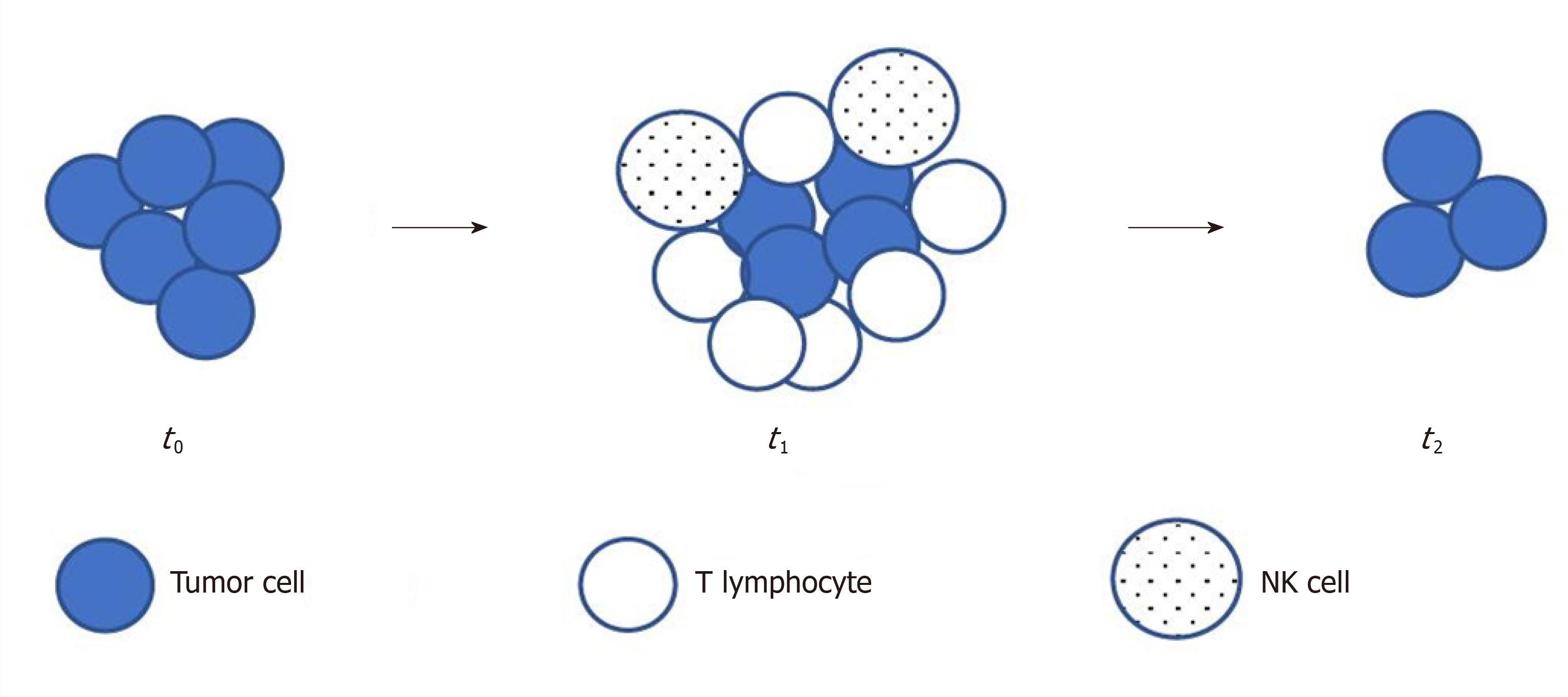
Frontiers | Investigation of the efficacy and safety of cryoablation and intra-arterial PD-1 inhibitor in patients with advanced disease not responding to checkpoint inhibitors: An exploratory study
Physical exercise during neoadjuvant chemotherapy for breast cancer as a mean to increase pathological complete response rates: Trial protocol of the randomized Neo-ACT trial | PLOS ONE