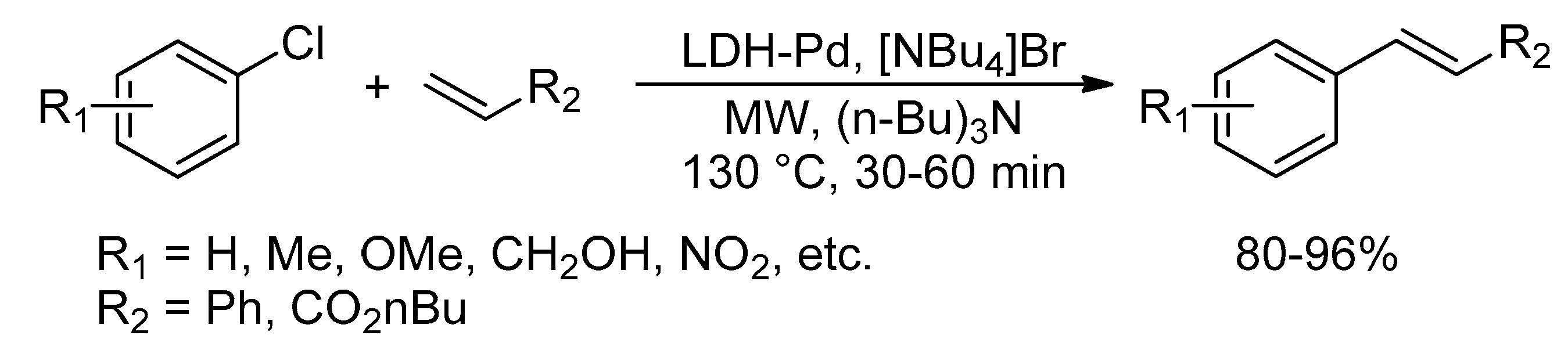
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Mixed-Ligand Approach to Palladium-Catalyzed Direct Arylation Polymerization: Synthesis of Donor–Acceptor Polymers Containing Unsubstituted Bithiophene Units - Macromolecules - X-MOL
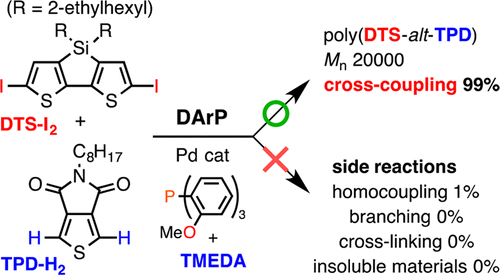
Mixed-Ligand Approach to Palladium-Catalyzed Direct Arylation Polymerization: Effective Prevention of Structural Defects Using Diamines - Macromolecules - X-MOL

Table 1 from An efficient diamine.copper complex-catalyzed coupling of arylboronic acids with imidazoles | Semantic Scholar

Pd-catalyzed synthesis of α,β-unsaturated ketones by carbonylation of vinyl triflates and nonaflates - Chemical Communications (RSC Publishing)

Iron-catalysed cross-coupling of organolithium compounds with organic halides | Nature Communications
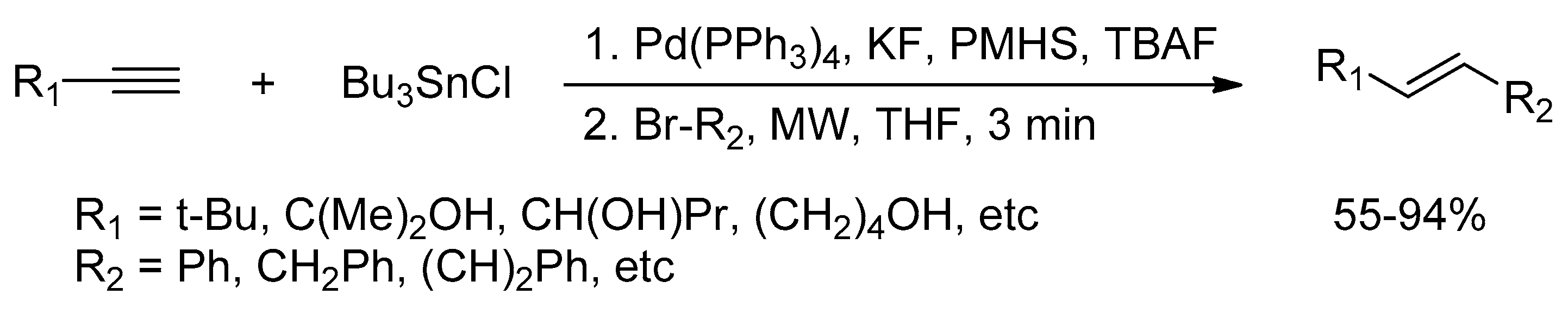
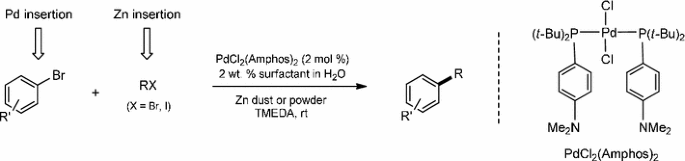
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

One-pot synthesis of self-assembled heteroleptic palladium(II) complexes with tmeda: An application of ligand exchange reactions - ScienceDirect
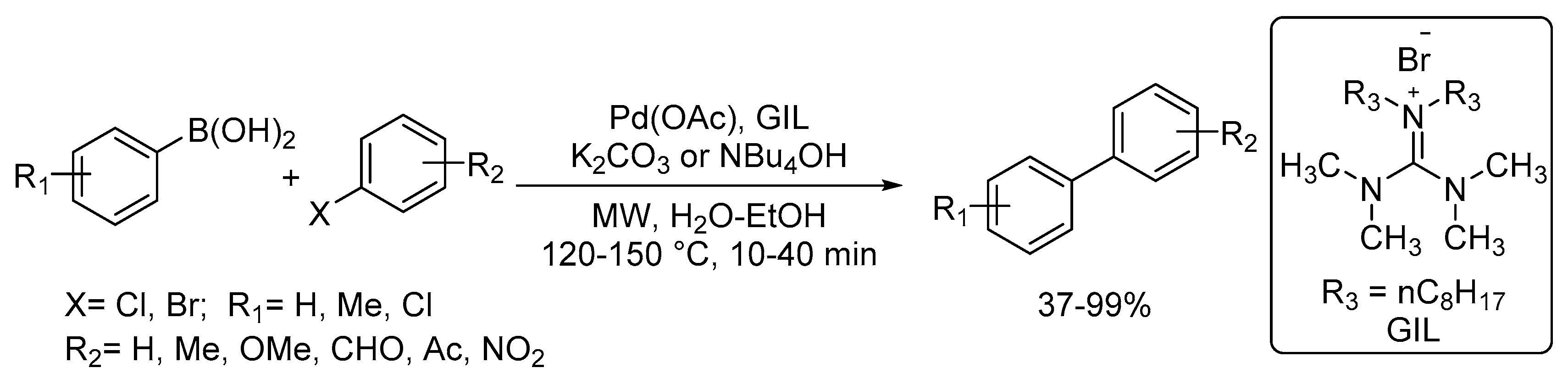
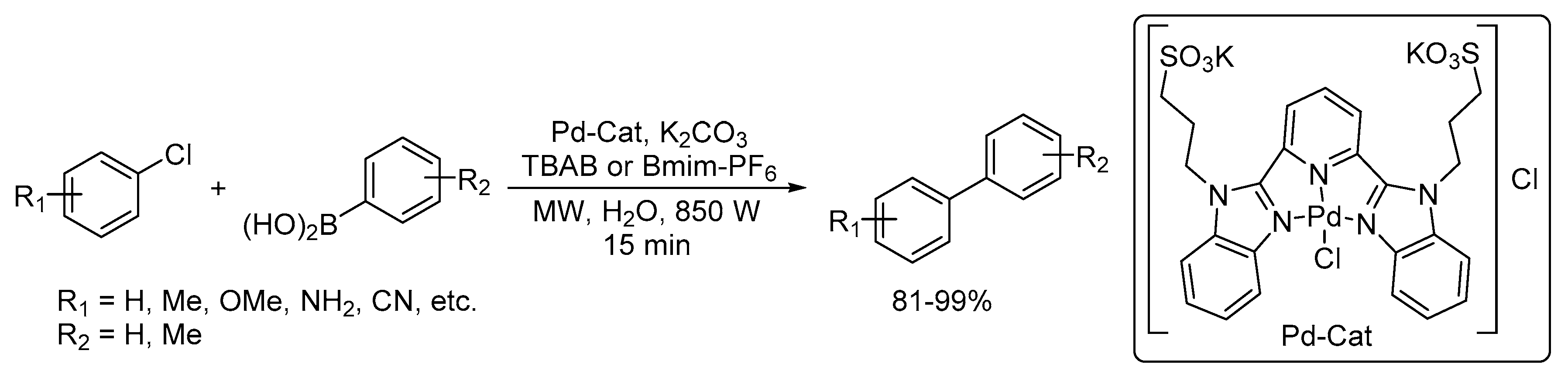
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Figure 1 from Palladium-catalyzed enantioselective alpha-arylation and alpha-vinylation of oxindoles facilitated by an axially chiral P-stereogenic ligand. | Semantic Scholar

Optimization of palladium-catalyzed Ferrier-type C-glycosylation with... | Download Scientific Diagram
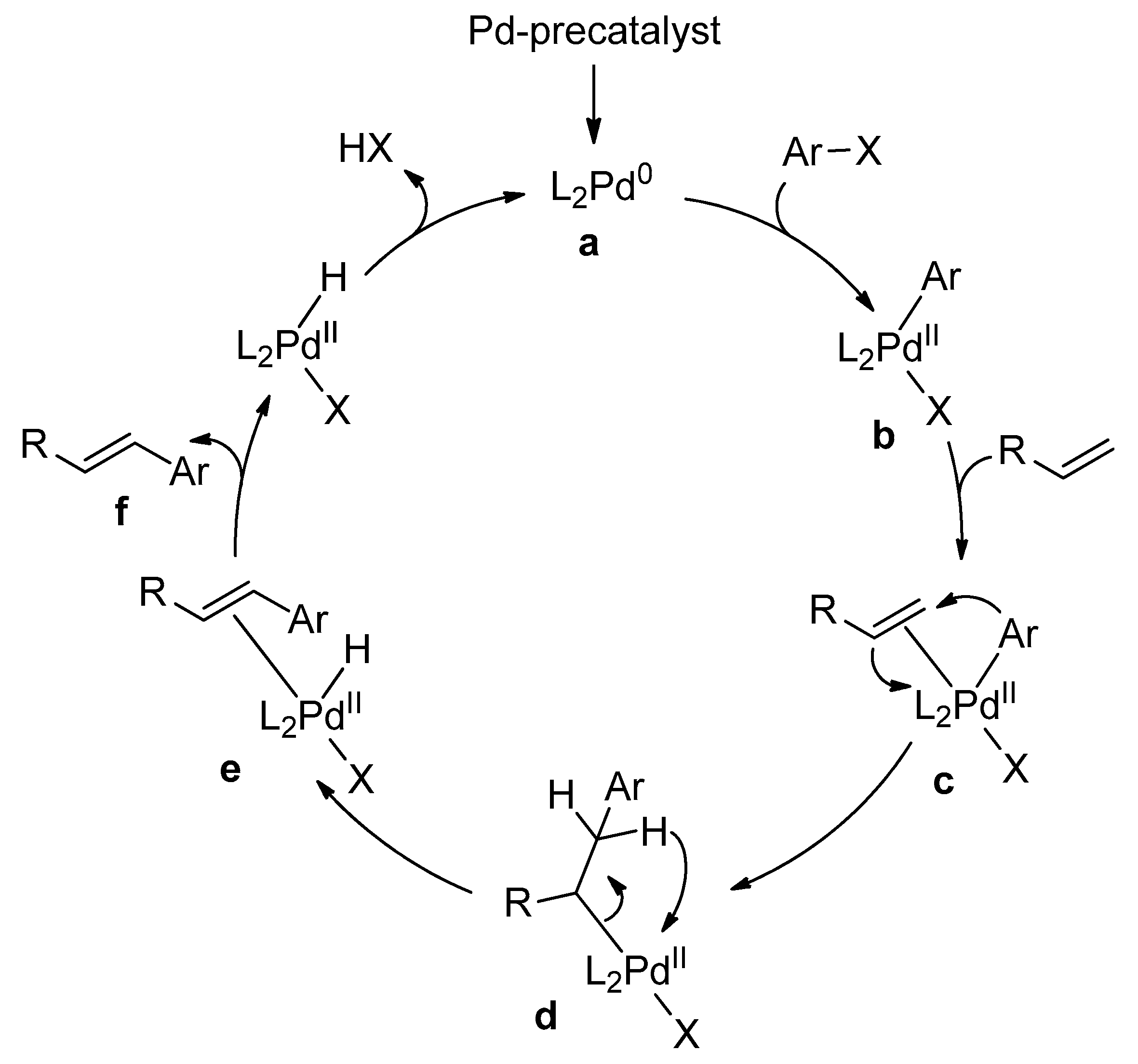
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

Hydrodehalogenation of halogenated pyridines and quinolines by sodium borohydride/N,N,N′,N′-tetramethylethylenediamine under palladium catalysis - ScienceDirect

NaBH4-TMEDA and a palladium catalyst as efficient regio- and chemoselective system for the hydrodehalogenation of halogenated heterocycles - ScienceDirect
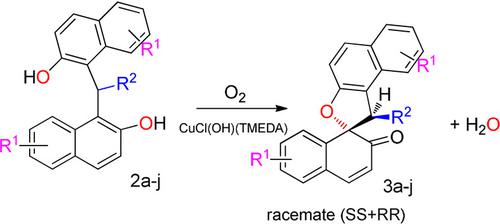
Aerobic Copper Catalytic Oxidation of Methylene and Arylidenebisnaphthols: A Green and Efficient Synthesis of Spironaphthalenones - ChemistrySelect - X-MOL

Palladium-catalyzed asymmetric dearomative alkenylation of indoles through a reductive-Heck reaction - Organic Chemistry Frontiers (RSC Publishing)

EP2107047A1 - Method for producing organic compounds by means of a transition metal-catalysed cross-coupling reaction of an aryl-X, heteroaryl-X, cycloalkenyl-X or alkenyl-X compound with an alkyl, alkenyl, cycloalkyl or cycloalkenyl halogenide -