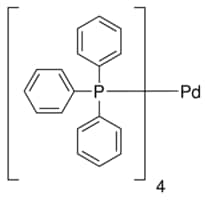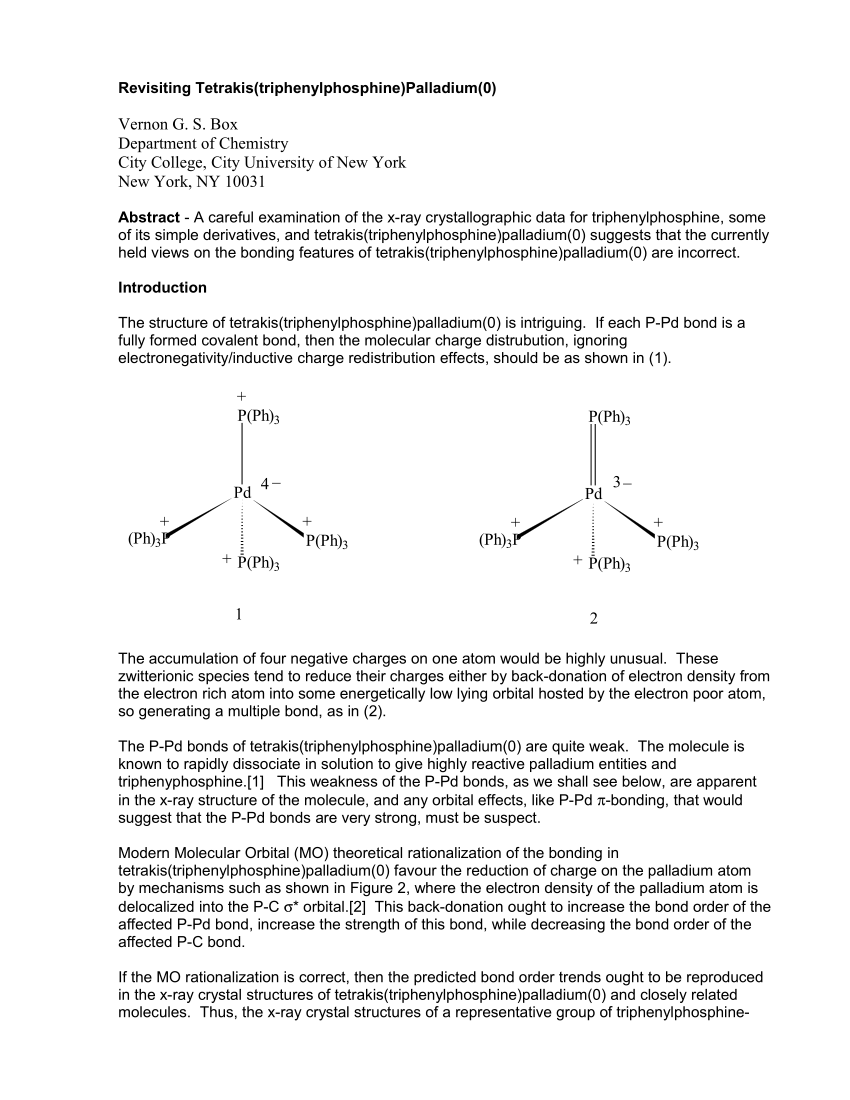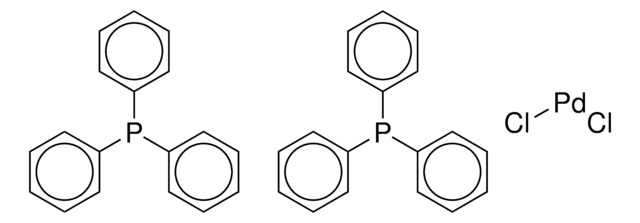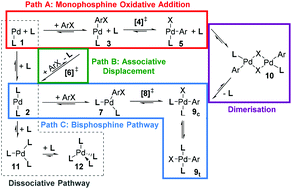
Computed ligand effects on the oxidative addition of phenyl halides to phosphine supported palladium(0) catalysts - Dalton Transactions (RSC Publishing)
![Molecules | Free Full-Text | Syntheses, Reactivities, Characterization, and Crystal Structures of Dipalladium Complexes Containing the 1,3-pyrimidinyl Ligand: Structures of [Pd(PPh3)(Br)]2(μ,η2-C4H3N2)2, [Pd(Br)]2(μ,η2-Hdppa)2, and [{Pd(PPh3)(CH3CN)}2 ... Molecules | Free Full-Text | Syntheses, Reactivities, Characterization, and Crystal Structures of Dipalladium Complexes Containing the 1,3-pyrimidinyl Ligand: Structures of [Pd(PPh3)(Br)]2(μ,η2-C4H3N2)2, [Pd(Br)]2(μ,η2-Hdppa)2, and [{Pd(PPh3)(CH3CN)}2 ...](https://www.mdpi.com/molecules/molecules-25-02035/article_deploy/html/images/molecules-25-02035-g001.png)
Molecules | Free Full-Text | Syntheses, Reactivities, Characterization, and Crystal Structures of Dipalladium Complexes Containing the 1,3-pyrimidinyl Ligand: Structures of [Pd(PPh3)(Br)]2(μ,η2-C4H3N2)2, [Pd(Br)]2(μ,η2-Hdppa)2, and [{Pd(PPh3)(CH3CN)}2 ...
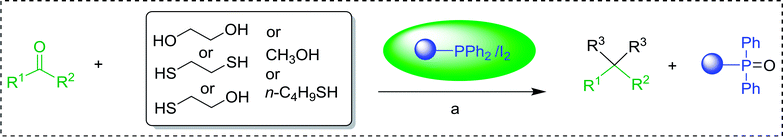
Polymer-supported triphenylphosphine: application in organic synthesis and organometallic reactions - RSC Advances (RSC Publishing) DOI:10.1039/C9RA07094J

Crystal structure of a novel polymorph of trans-dichlorobis ( triphenylphosphine) palladium (II) and its application as a novel, efficient and retrievable catalyst for the amination of aryl halides and stille cross-coupling reactions -
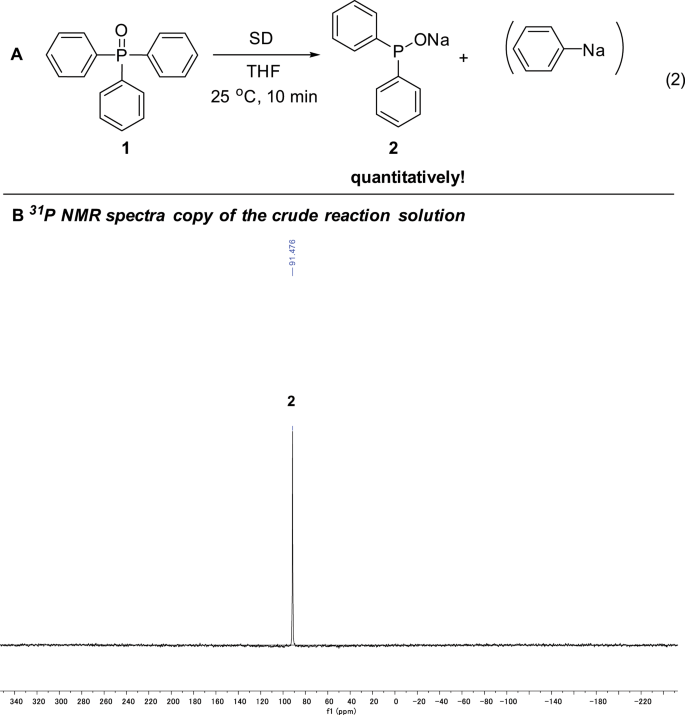
Conversion of triphenylphosphine oxide to organophosphorus via selective cleavage of C-P, O-P, and C-H bonds with sodium | Communications Chemistry
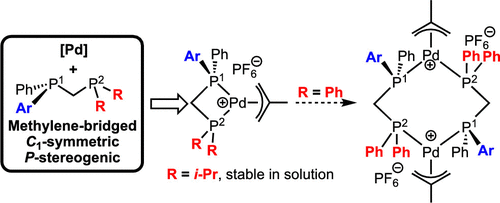
Palladium Complexes of Methylene-Bridged P-Stereogenic, Unsymmetrical Diphosphines - Organometallics - X-MOL
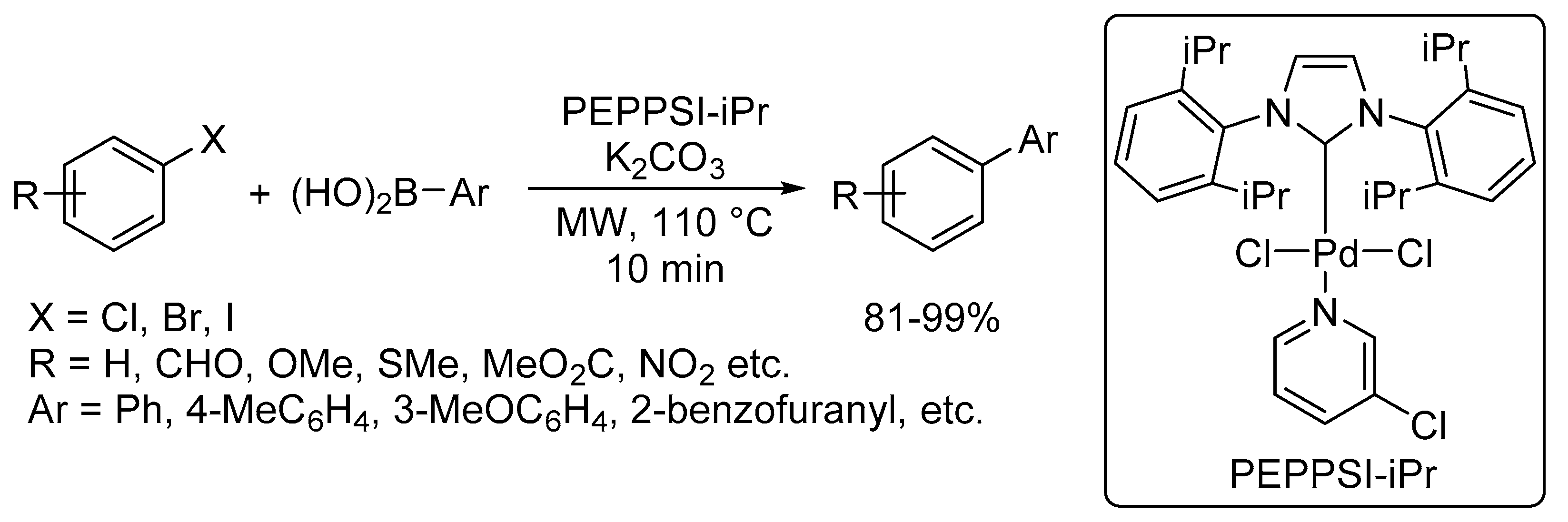
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

The Palladium Acetate‐Catalyzed Microwave‐Assisted Hirao Reaction without an Added Phosphorus Ligand as a “Green” Protocol: A Quantum Chemical Study on the Mechanism - Keglevich - 2017 - Advanced Synthesis & Catalysis -
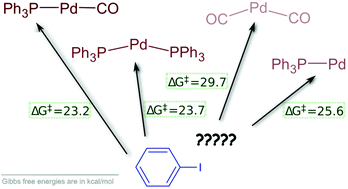
Viable pathways for the oxidative addition of iodobenzene to palladium(0)- triphenylphosphine-carbonyl complexes: a theoretical study - Dalton Transactions (RSC Publishing)
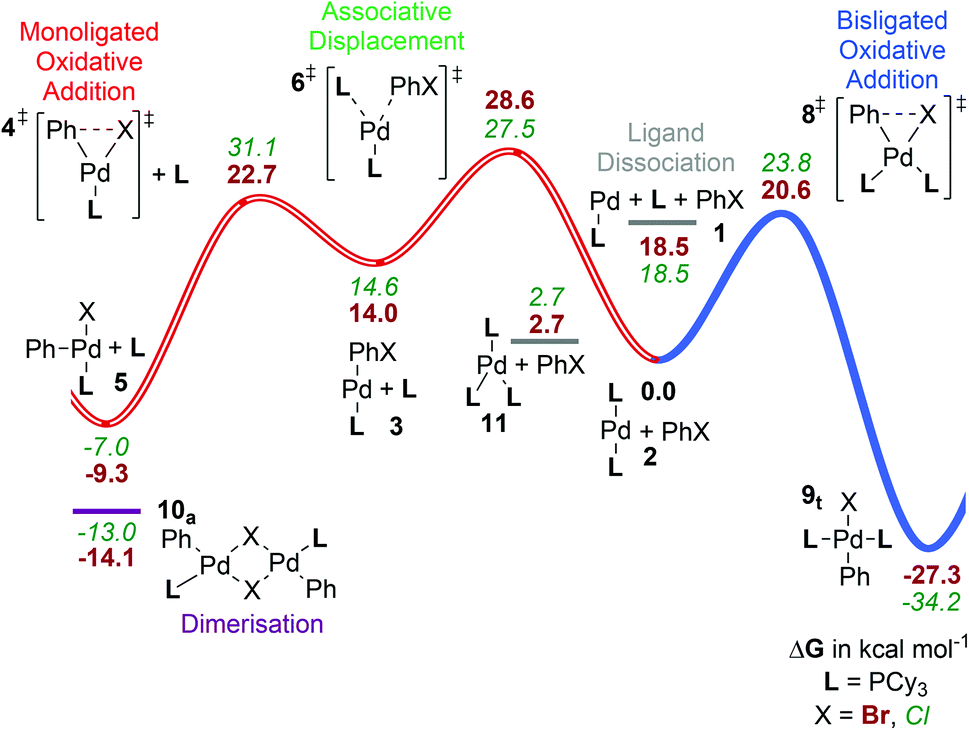
Computed ligand effects on the oxidative addition of phenyl halides to phosphine supported palladium(0) catalysts - Dalton Transactions (RSC Publishing)